Log in
Search
Latest topics
» Tee Dee .020 combat modelby TD ABUSER Today at 12:38 am
» My latest doodle...
by batjac Yesterday at 9:47 pm
» My N-1R build log
by roddie Yesterday at 8:50 pm
» Free Flight Radio Assist
by rdw777 Yesterday at 4:51 pm
» Purchased the last of any bult engines from Ken Enya
by getback Yesterday at 12:05 pm
» Funny what you find when you go looking
by rsv1cox Wed Nov 20, 2024 3:21 pm
» Landing-gear tips
by 1975 control line guy Wed Nov 20, 2024 8:17 am
» Cox NaBOO - Just in time for Halloween
by rsv1cox Tue Nov 19, 2024 6:35 pm
» Canada Post strike - We are still shipping :)
by Cox International Tue Nov 19, 2024 12:01 pm
» Duende V model from RC Model magazine 1983.
by getback Tue Nov 19, 2024 6:08 am
» My current avatar photo
by roddie Mon Nov 18, 2024 9:05 pm
» Brushless motors?
by rsv1cox Sun Nov 17, 2024 6:40 pm
Cox Engine of The Month
Diy Anodizing
Page 2 of 3
Page 2 of 3 •  1, 2, 3
1, 2, 3 
 Re: Diy Anodizing
Re: Diy Anodizing
i did it alredy crome and Chrome plated is a dangerous thing and one must take extreme care its very hard To machine i use metal nickel Not coper nickel aloy acétate What are the uses of Nickel?
Nickel has been a favored component of coins because it is bright and takes a fine polish and because it is lighter than copper, silver and other metals commonly used in coin currency. In 1850, Switzerland became the first modern nation to officially employ nickel in its coinage. The U.S. soon followed suit in the 1850s and ’60s when it introduced nickel to its penny and five-cent pieces to make them lighter. Though the U.S. five-cent coin only contained 25 percent nickel, it quickly became known as “the nickel.” The first pure nickel coin was issued by Switzerland in 1881; Austria and Hungary followed suit in 1893.
Because nickel does not easily oxidize, or rust, the metal was adapted as an electroplating material in the 1850s. Electroplating is a process in which metal ions in a chemical solution are attracted to a solid metal electrode. As the ions bind to the surface of the metal they form a uniform, thin coating. Electroplating a metal surface with nickel can form a layer that protects against corrosion. As the electrochemistry of nickel became better understood, it was adapted for use in batteries. Today nickel and cadmium compounds are used to produce rechargeable nickel-cadmium (Ni-Cd) batteries.
By far the largest use of nickel today is in the steel industry, which uses approximately two-thirds of the world’s annually produced nickel. The metal has the unusual properties of being hard — strong, able to withstand breaking under high forces — and ductile — able to yield or bend before breaking or cracking. In addition, nickel is chemically similar to iron but with particularly good resistance to oxidation. Because of its similarity to iron, nickel can readily substitute for iron in steel alloys or mixtures. The addition of nickel to steel increases its strength, ductility, its rust resistance and its value.
So-called stainless steels, which contain chromium and between 5 and 25 percent nickel,
Nickel has been a favored component of coins because it is bright and takes a fine polish and because it is lighter than copper, silver and other metals commonly used in coin currency. In 1850, Switzerland became the first modern nation to officially employ nickel in its coinage. The U.S. soon followed suit in the 1850s and ’60s when it introduced nickel to its penny and five-cent pieces to make them lighter. Though the U.S. five-cent coin only contained 25 percent nickel, it quickly became known as “the nickel.” The first pure nickel coin was issued by Switzerland in 1881; Austria and Hungary followed suit in 1893.
Because nickel does not easily oxidize, or rust, the metal was adapted as an electroplating material in the 1850s. Electroplating is a process in which metal ions in a chemical solution are attracted to a solid metal electrode. As the ions bind to the surface of the metal they form a uniform, thin coating. Electroplating a metal surface with nickel can form a layer that protects against corrosion. As the electrochemistry of nickel became better understood, it was adapted for use in batteries. Today nickel and cadmium compounds are used to produce rechargeable nickel-cadmium (Ni-Cd) batteries.
By far the largest use of nickel today is in the steel industry, which uses approximately two-thirds of the world’s annually produced nickel. The metal has the unusual properties of being hard — strong, able to withstand breaking under high forces — and ductile — able to yield or bend before breaking or cracking. In addition, nickel is chemically similar to iron but with particularly good resistance to oxidation. Because of its similarity to iron, nickel can readily substitute for iron in steel alloys or mixtures. The addition of nickel to steel increases its strength, ductility, its rust resistance and its value.
So-called stainless steels, which contain chromium and between 5 and 25 percent nickel,
Last edited by davidll1984 on Mon Mar 15, 2021 10:04 am; edited 1 time in total

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan
 Re: Diy Anodizing
Re: Diy Anodizing
Lots of new stuf i have test grade 3 anodisation army spec.Almost as hard as diamond i use more volt To amp wit ice cold électrolyte the dark finish is very scratch résistent The piece of aluminum that I used wears against the anodize same wit dril bit made very hard Not the best color but how strong it become je just impossible 

Is the result of the blue parts instal on one custom modified performance engine look Good



Is the result of the blue parts instal on one custom modified performance engine look Good

Last edited by davidll1984 on Mon Mar 15, 2021 10:48 am; edited 1 time in total

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

rsv1cox- Top Poster




Posts : 11245
Join date : 2014-08-18
Location : West Virginia
 Re: Diy Anodizing
Re: Diy Anodizing
Wel i have To Improve my equipment before taking contracts for others that would please me To make parts for al of you that like m'y job Thank you i want To build m'y own modified models rc engine ad som new cool color The original colors of some engine y have lots of test just like wit the cars y build He must all pass a series of tests Everything like the biggest company To I have to make sure of the quality Before sell anything Or even before doing it for free if Not color as Planned y have consistent
result wit red blue the new color switch Not so its posible To make only Once the nex one mabe difrent i have lots of fun
result wit red blue the new color switch Not so its posible To make only Once the nex one mabe difrent i have lots of fun

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan
 Re: Diy Anodizing
Re: Diy Anodizing
Look Good i made clutch flyweel To fit m'y new tune pipe color 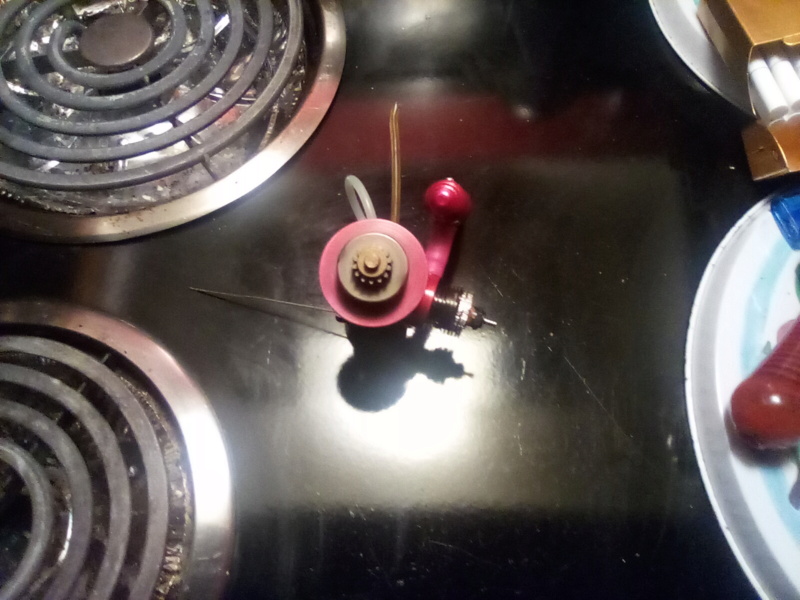

two color on the flywheel hard To see one side red On the other side a little bit of purple the red color stain the other side and the two color mix To difrent color Not To mutch difrent from m'y tuned pipe the exaust collector is exact same color as the pipe


two color on the flywheel hard To see one side red On the other side a little bit of purple the red color stain the other side and the two color mix To difrent color Not To mutch difrent from m'y tuned pipe the exaust collector is exact same color as the pipe

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

rsv1cox- Top Poster




Posts : 11245
Join date : 2014-08-18
Location : West Virginia

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan
 Re: Diy Anodizing
Re: Diy Anodizing
A great job well done David. You sure seem to have the hang of this now.

NEW222- Top Poster

- Posts : 3896
Join date : 2011-08-13
Age : 46
Location : oakbank, mb
 Re: Diy Anodizing
Re: Diy Anodizing
With the current temperature it works very well neither too hot nor too cold just perfect i am currently making a batch of blue parts for .09 tee dee cl engine 



davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan
 Re: Diy Anodizing
Re: Diy Anodizing
Dear David, I come in silence following your anodizing progress and you are really achieving very good finishes.
Maybe in a few days I will try to do something here with some pieces that I have, so ... do you dare to make us an instruction manual to follow to achieve those results?
for example:
Necessary materials
Temperatures and procedures per step.
It would be very very interesting to be able to do this.
My sincere thanks in advance
Maybe in a few days I will try to do something here with some pieces that I have, so ... do you dare to make us an instruction manual to follow to achieve those results?
for example:
Necessary materials
Temperatures and procedures per step.
It would be very very interesting to be able to do this.
My sincere thanks in advance

MauricioB- Top Poster

- Posts : 3712
Join date : 2016-02-16
Age : 53
Location : ARG

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan
 Re: Diy Anodizing
Re: Diy Anodizing
At the beginning the part must be ultra clean
For the mixture of acid and water 1 \ 4 water wit acid just acid is ok wil work Sometimes the part takes longer than others take the time dont try boost volt amp Unless you are able to keep the acid under 0-3 'or less A missed part is very difficult to correct Monitor the temperature The piece anodize seal with heat try To not let parts out the water To long if very hot in the place

For the mixture of acid and water 1 \ 4 water wit acid just acid is ok wil work Sometimes the part takes longer than others take the time dont try boost volt amp Unless you are able to keep the acid under 0-3 'or less A missed part is very difficult to correct Monitor the temperature The piece anodize seal with heat try To not let parts out the water To long if very hot in the place

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan

davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan
 Re: Diy Anodizing
Re: Diy Anodizing
Once the parts are ready Rinse the parts in distilled water To rinse it well, use two containers now Dip dab draw the color it's up to you After the desired color is reached Let the piece sit for a little while
Now heat the water very close to the boiling point Soaking the parts in hot water for a few seconds is enough To see if it is successful Or not if this part discolored it is not a succest and its It is sometimes very hard to start from the beginning.Sometimes the faults remain after second atempt

Now heat the water very close to the boiling point Soaking the parts in hot water for a few seconds is enough To see if it is successful Or not if this part discolored it is not a succest and its It is sometimes very hard to start from the beginning.Sometimes the faults remain after second atempt


davidll1984- Diamond Member

- Posts : 2327
Join date : 2020-02-12
Age : 39
Location : shawinigan
 Re: Diy Anodizing
Re: Diy Anodizing
David, j'espère que ce fil durera encore un peu. Tu
semblait avoir vraiment bien affiné le processus. J'espère
essayez moi-même. Merci!
Bob
David, I hope this thread goes on for a while longer. You
seemed to have really refined the process well. I hope to
give it a try myself. Thanks!
Bob
semblait avoir vraiment bien affiné le processus. J'espère
essayez moi-même. Merci!
Bob
David, I hope this thread goes on for a while longer. You
seemed to have really refined the process well. I hope to
give it a try myself. Thanks!
Bob

dckrsn- Diamond Member

- Posts : 2750
Join date : 2010-10-21
Age : 71
Location : Long Island, New York
 Re: Diy Anodizing
Re: Diy Anodizing
David, where do you buy your battery acid from up here in Canada? Just curious as I do think I want to try this while on holidays in a few weeks and would like to get my supplies ready. And as for color using bingo dabbers. Do you use full strength, or do you dilute the bingo dabber ink with water as well? If I am reading this correctly, is it 1 (one) part battery acid to 4 (four) parts water? Thank you.

NEW222- Top Poster

- Posts : 3896
Join date : 2011-08-13
Age : 46
Location : oakbank, mb
Page 2 of 3 •  1, 2, 3
1, 2, 3 
Page 2 of 3
Permissions in this forum:
You cannot reply to topics in this forum

 Rules
Rules






























